Abstract
Background: Apatinib, a small molecule vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase inhibitor, is a first-generation oral antiangiogenesis drug approved in the People's Republic of China for use as a subsequent line of treatment for advanced gastric cancer, also have shown remarkable efficacy in many solid cancers. But evidence of antitumor activity in patients with Lymphoma is still limited. We conducted an open-label, single-armed, exploratory study in relapse or refractory non-hodgkin lymphoma patients for the efficacy and safety of apatinib.
Methods: Patients age between 18 and 70 years with histologically confirmed non-hodgkin lymphoma and had to have taken at least two lines of chemotherapy fail before participating in the study were eligible for enrollment. Additional enrollment criteria were as follows: at least one measurable lesion, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and acceptable organ function. All eligible patients took a treatment comprised of oral apatinib 500mg once daily with 21 days as a treatment cycle. Treatment interruption resulting from toxicities was allowed for no more than 7 days (either continuously or cumulatively) in each treatment cycle. Efficacy was evaluated every two cycles. The primary end point was response rate. Secondary end points included progression-free survival (PFS) and overall survival (OS).
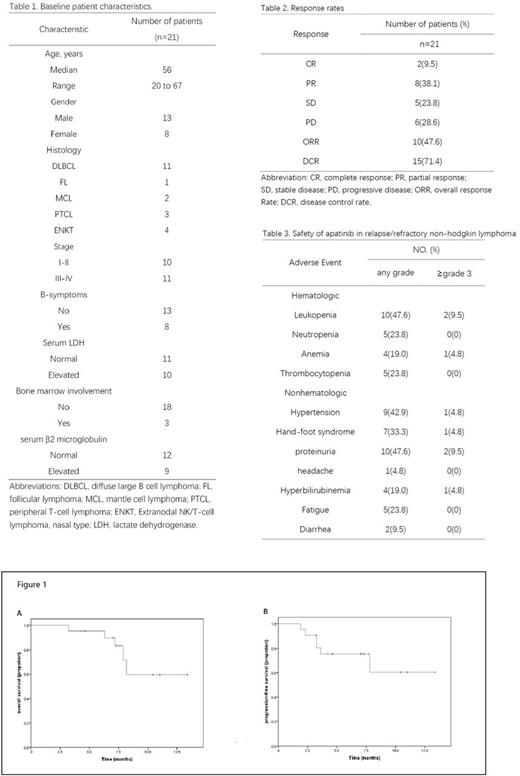
Results: Between February 2016 and December 2016, 21 patients were enrolled. Baseline clinical characteristics are listed in Table 1. The ORR (CR plus PR) was 47.6% (10 of 21 patients) included 9.5% CRs and 38.1% PRs. 23.8% patients achieved stable disease made the DCR 71.4% (15/21) (Table 2). Response rates by histology were: DLBCL, 6 of 11 patients; MCL, one of two; FL, one of one; ENKT, one of four; and PTCL, two of three. The median OS was 7.3 months (95% CI, 7.1 to 7.9) and the median PFS was 7.1 months (95% CI, 4.2 to 7.3). Adverse events (AEs) are listed in Table 3. Most patients suffered from grade 1 to grade 2 treatment-related adverse events. Dose modifications resulting from toxicity occurred on two patients for hand-foot syndrome and proteinuria, respectively. Death resulting from toxicity was not observed. The most common nonhematologic adverse events were proteinuria (47.6%), hypertension (42.9%) and hand-foot syndrome (33.3%).
Conclusions: In our study, the results we presented showed the VEGFR-TKI apatinib might have a rapid, safe and high efficacy on relapsed or refractory non-Hodgkin lymphoma patients. And more clinic trials are expected to be taken to identification the efficacy of apatinib on lymphoma further.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal